Since December 1, 2025, shares of Alpha Tau Medical (NASDAQ: DRTS) have climbed by nearly 85%, pushing the company’s market capitalization to just under $600 million. The move follows a steady drumbeat of clinical updates, new U.S. trial authorizations, and regulatory progress across multiple cancer indications - momentum that has brought renewed attention to the company’s core technology. At the heart of Alpha Tau’s story is Alpha DaRT, a radiation therapy designed to solve one of oncology’s oldest problems: how to destroy tumors effectively without inflicting significant damage on the healthy tissue around them.
Radiation has been used to treat cancer for over a century, but it comes with a fundamental tradeoff: the same energy that kills tumors can damage healthy tissue nearby. External beam radiation fires gamma rays or X-rays through the body from outside, hoping to hit the tumor while limiting collateral damage. The longer range of these radiation types means healthy cells inevitably get caught in the crossfire.
Alpha Tau Medical (NASDAQ: DRTS) has developed a different approach. Instead of blasting radiation from outside, its Alpha DaRT technology implants tiny radioactive seeds directly into tumors. These seeds release alpha particles, a form of radiation that travels only a very short distance before stopping. The result is a highly localized treatment that aims to destroy the tumor while leaving surrounding tissue largely unaffected.
The Power and Limitation of Alpha Particles
Gamma rays and X-rays can travel considerable distances through the body, depositing energy along their entire path and damaging healthy cells along the way. Beta particles, used in some targeted therapies, travel several millimeters, which can still damage cells beyond the target.
Alpha particles are different. Made up of two protons and two neutrons, they carry a double positive charge and interact strongly with matter, depositing all their energy in just 50 to 90 micrometers—roughly the width of a few cells. Per unit of distance traveled, alpha particles transfer far more energy than gamma or beta radiation, a property known as high linear energy transfer.
As a result, alpha particles are significantly more biologically effective at killing cells than conventional radiation. A single alpha particle passing through a cell nucleus can cause multiple double-strand breaks in DNA, damage that is particularly difficult for cells to repair. Unlike gamma radiation, which relies on oxygen to maximize its effect, alpha particles work equally well in oxygen-deprived tumor cores.
The catch is that alpha particles travel such a short distance that delivering them to a tumor has historically been the challenge. They cannot be fired through the bo
dy like X-rays, as they would stop in the first few cells they encounter.
How Alpha DaRT Solves the Range Problem
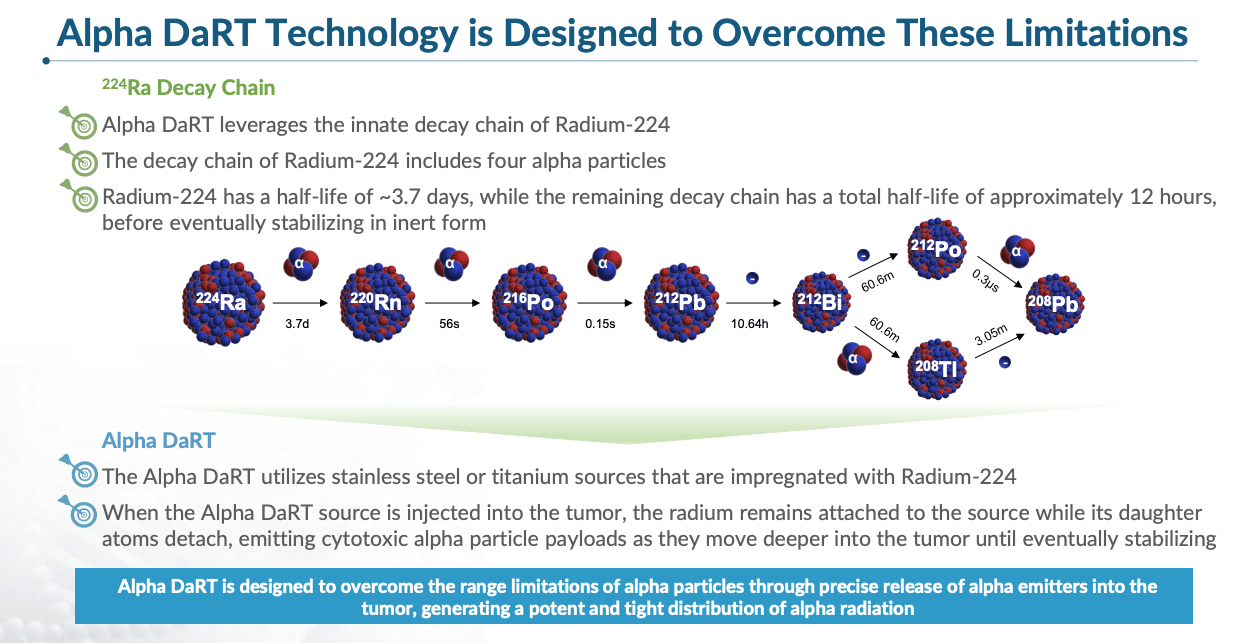
Alpha Tau’s solution is to deliver alpha particles from inside the tumor using tiny seeds coated with radium-224.
Radium-224 has a half-life of about 3.6 days. When it decays, it releases radon-220, a noble gas that diffuses locally through tumor tissue, emitting alpha particles as it decays further. The decay chain continues, producing additional short-lived alpha-emitting atoms that spread through the tumor.
Think of it like a slow-release mechanism. Each seed continuously releases radioactive atoms that spread locally through the tumor over several millimeters, emitting alpha particles along the way and creating a high-dose “kill region” around each seed. By spacing multiple seeds a few millimeters apart - often less than 5 millimeters between sources - clinicians can cover the entire target area of the tumor while maintaining a sharp dose drop-off outside the treatment zone.
The treatment itself is straightforward. For superficial tumors, seeds are inserted under local anesthesia as an outpatient procedure. For internal tumors, such as pancreatic cancer, endoscopic ultrasound is used to guide placement. After approximately 14 to 21 days, once the radiation dose has largely been delivered, the seeds are removed.
How Alpha DaRt overcomes the limitations of Alpha Particles
Why This Approach May Matter
The localized nature of alpha radiation means the dose falls off rapidly outside the treatment area. This could allow tumors to be treated in locations where conventional radiation risks damaging critical surrounding structures. The company is currently studying Alpha DaRT in recurrent glioblastoma, an aggressive brain cancer where sparing healthy tissue is essential. In December 2025, Alpha Tau treated the first patient in this trial at The Ohio State University, achieving greater than 95% tumor coverage.
Because alpha radiation does not depend on oxygen, it may be effective against hypoxic tumors that resist conventional therapies. Early clinical data also suggest Alpha DaRT may preserve immune function better than conventional radiation. Final results from a 32-patient pancreatic cancer study conducted in Montreal and released in January 2026 showed an 81% disease control rate, along with immune marker stability one month after treatment.
Where Alpha DaRT Stands Today
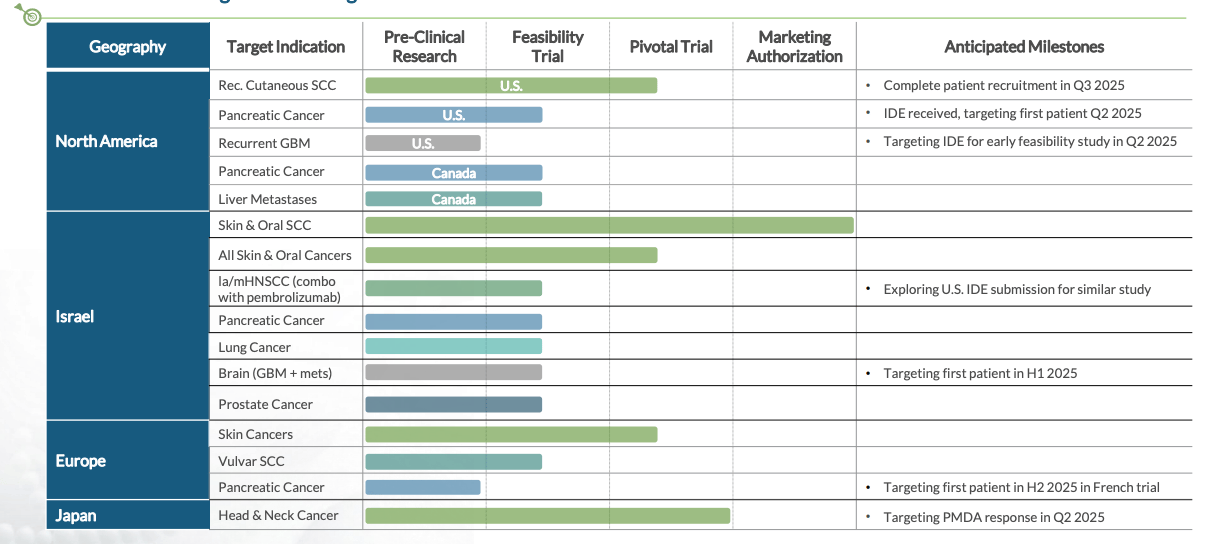
Alpha Tau reports that it now has five simultaneous active U.S. Investigational Device Exemptions (IDEs), the FDA pathway that allows clinical testing of a medical device in humans. Its most recent IDE, announced on December 2, 2025, supports a trial in locally recurrent prostate cancer. In January 2026, the company submitted the first module of its pre-market approval application for recurrent cutaneous squamous cell carcinoma.
Clinical data continue to accumulate. A U.S. multi-center pancreatic cancer pilot study is underway, with recruitment expected to be completed by the end of the first quarter of 2026. In head and neck cancer, a combination trial with pembrolizumab showed a 75% objective response rate in early patients, compared with a historical response rate of approximately 19% for pembrolizumab alone. Notably, The company ended the third quarter of 2025 with $75.9 million in cash and has secured a radioactive materials license for its New Hampshire manufacturing facility.
A Catalyst Rich Year Ahead
Alpha DaRT remains in clinical development, and regulatory approval is not guaranteed. But the underlying physics applies broadly: in theory, any solid tumor that can be accessed with a needle or scope could be treated. That is why Alph a Tau is advancing trials across skin, head and neck, pancreatic, brain, and prostate cancers simultaneously. With multiple proof-of-concept studies advancing in parallel and several high-impact data readouts expected over the next 12 months, 2026 is shaping up to be a potentially transformative year for the company.
Alpha Tau Medical’s Development Pipeline
If the data continue to hold up, Alpha Tau would not be building toward a single product approval, but toward a differentiated oncology platform with the potential to reshape how localized radiation is delivered.
Subscribe to Get our Exclusive Insights to your Inbox
=
Recent News Highlights from Alpha Tau
=
Legal Disclaimers, Terms & Disclosures:
By using CapNotes, any related brands thereof, and any affiliated or partner websites operated under the Wall Street Wire Network (collectively referred to as “Services”), you acknowledge that any and all Services provided are for informational and entertainment purposes only and do not constitute a recommendation for any particular stock, company, investment, commodity, security, transaction, or any other method of trading featured anywhere on CapNotes or affiliated platforms. CapNotes does not guarantee the accuracy, completeness, or timeliness of the information or Services provided. Views and opinions presented through the Services, whether expressed by contributors, columnists, external partners, or employees, are not specifically endorsed by CapNotes or the Wall Street Wire Network, and neither entity accepts responsibility or liability for any actions, financial or otherwise, taken directly or indirectly as a result of engaging with any of the Services offered.
CapNotes, its employees, operator, partners, affiliates, and any other representatives will not, either directly or indirectly, be held liable, accountable, or responsible in any capacity to you or to any third party for any errors, inaccuracies, or omissions from the Services, including but not limited to market quotes, rumors, unverified chatter, financial data, and news reports; for any interruptions, delays, or transmission errors affecting the availability or accuracy of the Services; or for any damages or losses arising from or related to the use of, reliance on, or inability to access the Services. Some content published by CapNotes may reference market rumors, speculative chatter, or unconfirmed reports. Readers should be aware that while such unofficial information may be associated with market volatility, price movements based on speculative or incomplete data are subject to change rapidly upon further clarification or the release of official news or filings.
CapNotes reserves the right at any time to modify any part of its Terms of Service or any portion of the Services, including but not limited to the removal or addition of content, features, contributors, or affiliated content providers, or the introduction of any associated fees or usage conditions. Such changes will take effect immediately upon their publication across the Services and will apply to all users from the time of posting. Please note that trading in foreign currencies, stocks, options, and other securities involves substantial risk and may result in significant financial losses. Neither CapNotes nor its staff recommends that you buy, sell, or hold any security, and no part of the Services constitutes personalized investment advice. All information provided through the Services is general commentary intended for informational and entertainment use only. CapNotes disclaims any liability for loss or damage, including without limitation any loss of capital, profit, or opportunity, that may arise directly or indirectly from use of or reliance on the information contained within the Services. We encourage all users to conduct their own due diligence and consult with a certified financial advisor or licensed professional before making any financial decisions.
Content produced by or for CapNotes may not be reproduced, republished in full, or redistributed in any form without prior written permission from CapNotes or the Wall Street Wire Network, as applicable.
This content is a form of paid promotional content and advertising. Wall Street Wire has receives cash compensation from Oramed Pharmaceuiticals Inc for promotional media services provided to Alpha Tau Medical Ltd which we continue to provide on an ongoing subscription basis. This content is for informational purposes only and does not constitute financial advice. Wall Street Wire is not a broker-dealer or investment adviser. Full compensation details and information regarding the operator of Wall Street Wire alongside the full disclaimers and disclosures this content is subject to are available wallstwire.ai/disclosures. We are not responsible for any price targets or market size figures that may be cited in this article nor do we endorse them, they are quoted based on publicly available news reports and additional price targets or figures may exist that may not have been quoted. Readers are advised to refer to the full reports mentioned on various systems and the disclaimers/disclosures they may be subject to. This article was not reviewed or approved by the issuer prior to publication and should not be considered an official communication by it.